Abstract
Introduction The combination of daratumumab (DARA), lenalidomide (Len) and dexamethasone (Dex) is an FDA approved therapy for older adults with newly diagnosed multiple myeloma (NDMM) based on the MAIA study. A less intense bortezomib (Bort) lenalidomide dexamethasone combination (VRD lite) is also used. We thought to investigate a personalized daratumumab based response adapted therapy for older adults with NDMM.
Methods Patients were required to have NDMM and were age over 65 and not deemed candidates for high dose chemotherapy and autologous stem cell transplant. Eligible patients received subcutaneous DARA and Dex (20 mg PO weekly) for 2 cycles: in the event of partial response or better, patient continued on DARA Dex per usual dose and schedule, in the event of less than a partial response, frailty adjusted dosing of Len or Bort was added to the usual DARA Dex regimen. The choice of Len or Bort was per the treating physician and informed by Ex Vivo drug sensitivity assay. Modified geriatric assessment (including IMWG frailty score, timed up and go) and bone marrow biopsy (for correlatives) were performed at screening as well as on C2D22. Capitalizing on this unique opportunity to examine the tumor and immune response to DARA Dex, additional correlative studies looking at single cell RNAseq and multi-parameter flow cytometry are included. The primary endpoint was best response per IMWG criteria. Secondary endpoints included PFS, ORR after 2 cycles of DARA Dex.
Result: Between November 2019 and July 2022 (the data cutoff date), 25 patients were enrolled. The median age was 79 years (range 71-88) and 13 were males (52%). Frailty score per IMWG were 0 (fit), 1 (unfit), 2+(frail) in 16%, 32%, and 52% of patients respectively. Timed up and go was prolonged (>10s) in 7 (35%) patients. High risk FISH (t(4;14) and del17p) was noted in 3 (12%) with an additional 12 patients (48%) with gain 1q/Amp1q. RISS3 was noted in 16% of patients.
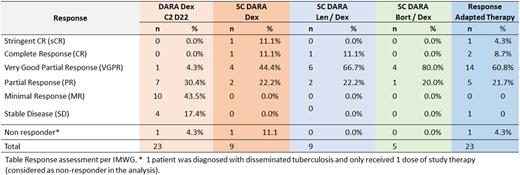
After 2 cycles of DARA Dex, 23 patients are evaluable for response (2 patients have not yet completed 2 cycles of therapy); 8 patients (35%) had a partial response or better (no patients had progressive disease, 1 patient discontinued therapy after 1 dose of study therapy and a diagnosis of disseminated tuberculosis). Of the 14 patients (61%) with less than a PR, 9 patients had len added and 5 bort added. The overall best response (PR and better) was 95% for this response adapted strategy including CR/sCR, VGPR, PR in 3 (13%), 14 (61%), 5 (21%) respectively. After a median of 14.7 months of follow up on this response adapted therapy, 5 had progressive disease, the estimated PFS at 1 year was 90% (95%CI 65-97.4).
Overall, no unexpected toxicity was noted. The most common grade 3+ AE at least possibly related to therapy included neutropenia, anemia, diarrhea, hyperglycemia, agitation and confusion and hypertension each occurring in 4% of patients. The most common possibly related any grade AE occurring in >25% of patients included hyperglycemia 80%, diarrhea 36%, neutropenia 28%, insomnia 28%, fatigue 28%
Conclusions: Preliminary results of this DARA based response adapted therapy are encouraging in this mostly frail patient population with a high response rate and low rate of severe AE. Enrollment in this response adapted therapy for older adults with NDMM continues. With longer follow-up, we will integrate correlative studies evaluating the accuracy of ex vivo predictions in response to DARA and combinations in this unique population of patients.
Disclosures
Baz:Shattuck labs: Membership on an entity's Board of Directors or advisory committees; genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; karyopharm: Research Funding; celgene: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding. Blue:Jassen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria; Sanofi: Consultancy, Speakers Bureau. Grajales-Cruz:sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Alsina:BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding. Shain:Bristol Myers Squibb (BMS), Janssen, GlaxoSmithKline (GSK), Adaptive, Sanofi, and Takeda, and Amgen: Honoraria; GSK, Janssen and BMS: Membership on an entity's Board of Directors or advisory committees; GSK, BMS, Sanofi, Karyopharm, Takeda, Janssen, Adaptive and Amgen: Speakers Bureau; AbbVie and Karyopharm: Research Funding; Janssen and BMS: Other: PI of clinical trials.
OffLabel Disclosure:
response adapted therapy for newly diagnosed myeloma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal